Stryker medical equipment
Stryker is an American company that has been developing innovative solutions for disposable products used in healthcare since 1971. Over the years, the company has gained the trust of leading healthcare facilities worldwide. Prevention is the most important pillar of the company’s philosophy. Preventing infections and other negative effects associated with healthcare in medical facilities is Stryker’s primary goal. In 2016, Stryker was acquired by Stryker Corporation, one of the leading companies in the medical technology industry, as part of its Medical Division. Stryker is a company that cares not only about the safety of patients but also of medical staff. It was the first to develop a medical device (sharps containers) made from recycled medical waste (earning the Green Cross certification in 1993) to ensure the safety of medical personnel.
Stryker Product Quality Guarantee
Drug and medical device manufacturers, such as Stryker, are required to adhere to numerous quality regulations and standards. The regulations enforced by Stryker include, among others, cGMP (current Good Manufacturing Practices) in the manufacturing, processing, packaging, or holding of drugs and finished pharmaceutical products, quality system regulations for medical devices, and electronic documentation. All Stryker products are manufactured in accordance with the applicable Good Manufacturing Practices (GMP) as required by the FDA (Food and Drug Administration). Stryker LLC has received accreditation from the Verified Accredited Wholesale Distributors (VAWD) program of the NABP, which aims to protect against counterfeit drugs.
Stryker’s Pioneering Products
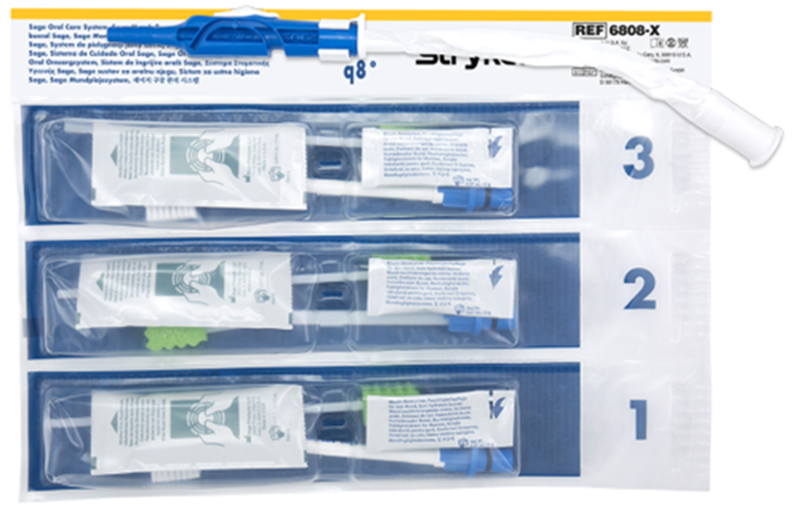
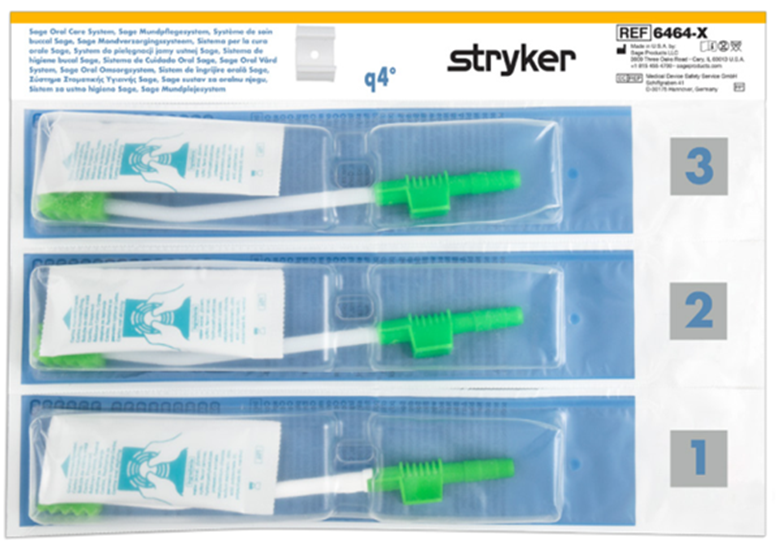
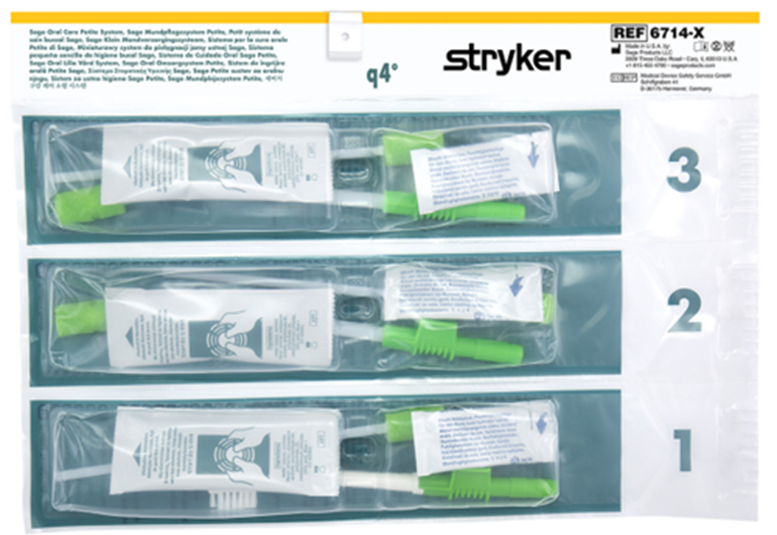
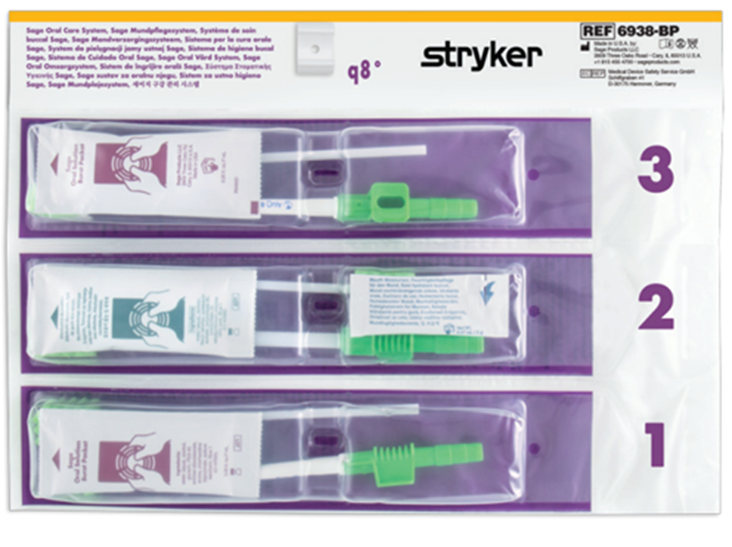
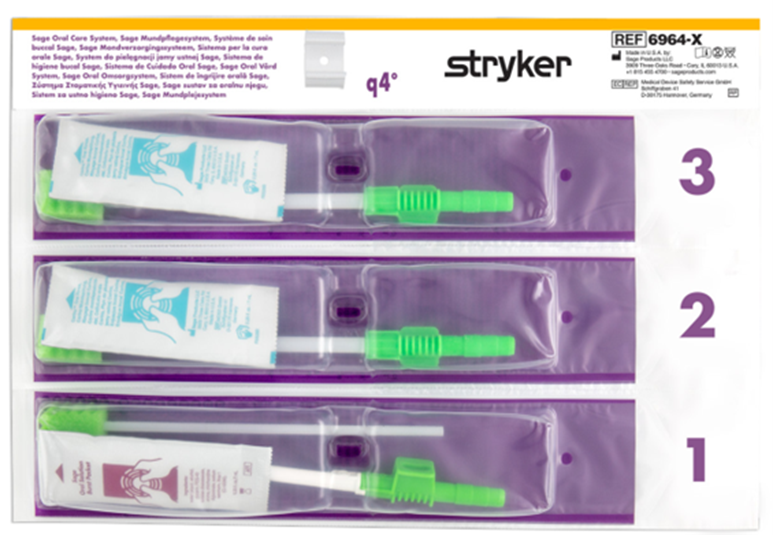
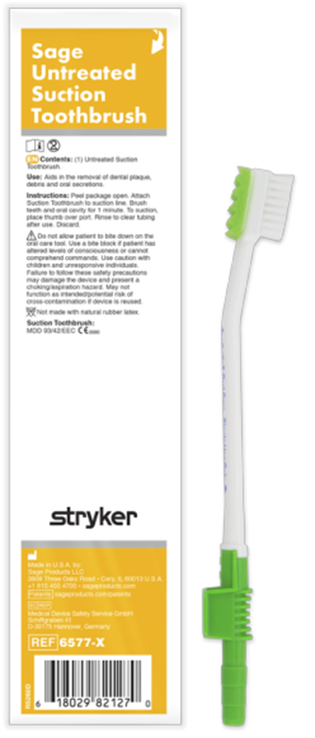
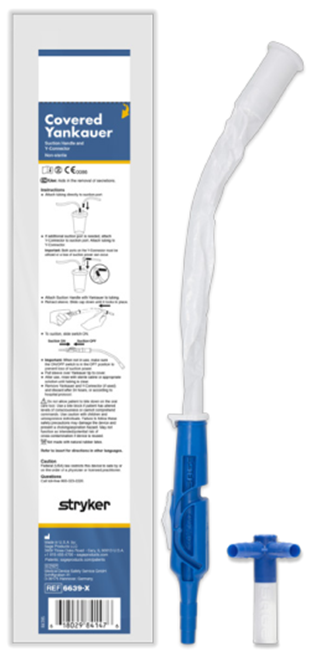
Stryker has developed a product portfolio aimed at infection prevention, including oral hygiene products designed to support the prevention of ventilator-associated pneumonia (VAP) and pressure ulcer prevention. Another product group is designed to prevent injuries to healthcare workers, such as the Prevalon positioning system, which ensures safe patient positioning. Prevalon products significantly reduce the physical strain on medical staff and minimize the physical effort required to change a patient’s position or transfer them.
Skamex is a distributor of Stryker medical products.
 Polski
Polski